Trump inks deal with Moderna for 100 million doses of COVID-19 vaccine
The deal with Moderna is part of the government “Operation Warp Speed,” which is the government’s push to get a vaccine available as quickly and safely as possible.
News 12 Staff
•
Aug 12, 2020, 1:26 PM
•
Updated 1,346 days ago
Share:
More Stories

Nobel in medicine goes to 2 scientists whose work enabled creation of mRNA vaccines against COVID-19
200ds ago1:25

Pharmacies say they have not received orders for newest COVID booster
204ds ago0:21

Biden administration announces $600M to produce COVID tests and will reopen website to order them
211ds ago0:41
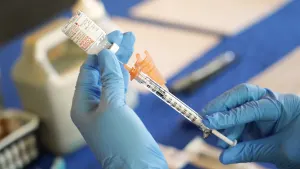
Gov. Hochul: Updated COVID-19 vaccine to be available in NY in the coming days
218ds ago2:13
NYSDOH: COVID numbers up with new variant accounting for 17% of new cases
252ds ago2:30
NYSHD: Hospitalizations caused by COVID increase by 22% in a week
260ds ago
Nobel in medicine goes to 2 scientists whose work enabled creation of mRNA vaccines against COVID-19
200ds ago1:25

Pharmacies say they have not received orders for newest COVID booster
204ds ago0:21

Biden administration announces $600M to produce COVID tests and will reopen website to order them
211ds ago0:41

Gov. Hochul: Updated COVID-19 vaccine to be available in NY in the coming days
218ds ago2:13

NYSDOH: COVID numbers up with new variant accounting for 17% of new cases
252ds ago2:30

NYSHD: Hospitalizations caused by COVID increase by 22% in a week
260ds agoAs News 12 has reported, Russia claims it has approved the first COVID-19 vaccine in the world, but there is skepticism about the product that came from a Moscow Institute. Meanwhile, work on U.S. vaccines continues.
The Trump administration has inked a $1.5 billion deal with Moderna Inc., one of the companies already producing a vaccine before proof it works.
"We're on track to rapidly produce 100 million doses as soon as the vaccine is approved and up to 500 million shortly thereafter," says President Donald Trump.
In an interview with CNN last month, principal investigator Dr. Paul Bradley says clinical trials are underway. Half of the subjects have the vaccine, the other half the placebo.
"Everyone gets treated as if they've got the same thing and we wait and we watch. We're looking for any side effects, any complaints," says Dr. Bradley.
The deal with Moderna is part of the government “Operation Warp Speed,” which is the government’s push to get a vaccine available as quickly and safely as possible.
"There's a process that takes place at the FDA, it's not one person who says 'We're good to go,' you know, it goes to a bunch of data scientists who look at the data, they compare it to other data and then they render these, you know, recommendations," says Dr. Sanjay Gupta, CNN Chief Medical Correspondent.
If testing goes well, experts say a vaccine will possible be available to the public early next year.
If testing goes well, experts say a vaccine will possible be available to the public early next year.
The World Health Organization says there are more than two-dozen COVID-19 vaccines that are in human trials.