More from News 12
0:49

Nassau County says it will rework its lease approval process in effort to build resort and casino
1:57
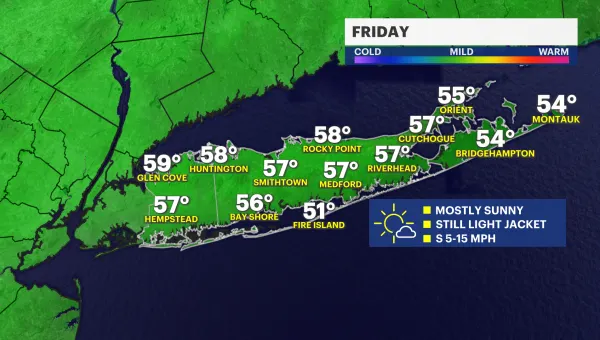
Sunny and cool for Friday; chance for rain late Saturday into Sunday
2:01

Students take part in pro-Palestinian protest at Hofstra University
1:53

Shop Mother’s Day Gifts – Exclusive Offers Up to 75% OFF!
1:56

Superintendent: Person accused of making threats to Islip School District in custody
1:06

South Setauket father charged for allegedly abusing infant son
1:27

East Meadow School District: Nesconset man accused of lewd act worked as social worker
2:35

Law enforcement resumes search in Manorville in connection with Gilgo Beach case
0:36

11 LIRR employees suspended without pay, accused of submitting fake COVID-19 vaccine cards
2:38
Islanders, fans gear up for Game 3 of playoffs at UBS Arena
2:05

Long Island Ducks to begin 2024 season tonight
1:13

The East End: Shou Sugi Ban House in Watermill
1:43

Have an out-of-this-world experience with the family at the Cradle of Aviation Museum

Ready to explore the great outdoors? These 14 tips can help you stay safe while hiking

Is your mom awesome? Long Island tell us why your Mom Rocks!
2:01

Police: 21-year-old woman fled fatal Massapequa DWI crash in stolen town patrol car
0:20

Police: Hempstead man killed in 3-car crash on Meadowbrook Parkway
0:21

UBS Arena to host 2024 MTV VMAs in September
0:26

Court adjourned for two suspects in human remains case
0:31
